What Are Good Sources of Protein? – Speed of Digestion
In the last article, What are good sources of protein – Digestibility, I examined some basics of protein digestibility and presented data on the gross digestibility of varying proteins. Summing up, animal source proteins such as meat, milk and eggs show extremely high (90%+) digestion while vegetable source proteins show much lower values.
However, the efficiency of digestion alone is not the only factor which goes into answering the question What are good sources of protein?
Recently (and by that I mean the late 90′s or so), an interest in the speed of digestion and how that impacts on various aspects of human physiology has occurred. It’s turning out that proteins can digest at fairly different rates and this turns out to affect various physiological processes; the main two are protein synthesis and protein breakdown. As with the last article, I’m going to talk about these terms in brief before moving onto the main thrust of today’s article.
Because I have a lot of information to cover, I’m going to break the topic down into two parts. In Part 1 today, I need to cover a bit more background physiology and talk about the original study that kicked off the entire interest in speed of digestion. In Part 2 (tomorrow), I’ll finish up with some other recent developments. As always, all of this information can be found in a more detailed and expanded fashion in The Protein Book.
Protein Turnover: The Combination of Protein Synthesis and Protein Breakdown
Early ideas about the body held that the different tissues such as fat cells and skeletal muscle (see What Does Body Composition Mean for a little more detail) were essentially static and unchanging. This turns out to be incorrect. At any given time in the body, pretty much all of the cells in your body are undergoing a constant process of breakdown (where larger structures are broken down into smaller) and synthesis (where smaller structures are combined to make larger).
So as you sit here reading this, your fat cells are both breaking down and re-synthesizing the triglyceride (fat) stored in them. Bone is undergoing the same constant processes as well. Of course, the same holds for protein tissues.
Right this moment, your liver is breaking down and remaking various proteins, and your skeletal muscle is in a constant source of breakdown and re-synthesis. While this is actually energetically very costly, and seems wasteful, it turns out to give the human body an incredible adaptability and ability to deal with stress.
The combination of breakdown and re-synthesis is referred to, generally, as turnover. In the context of protein based tissues, this is referred to as protein turnover.
I should note that different tissues in the body break down at drastically different rates. So while liver proteins may break down and be completely re-synthesized in a number of hours, skeletal muscle is turning over more slowly. Tissues such as organs, tendons and ligaments turn over much more slowly. As you’ll see, this actually has some implications for what I’m going to talk about in just a moment.
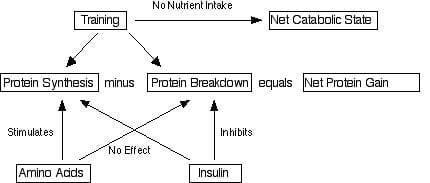
What happens overall to a given tissue (e.g. whether it grows, shrinks or stays the same) depends on the relative rate of synthesis and breakdown. Simply:
§ If synthesis is greater than breakdown, there will be an increase in the amount of that tissue.
§ If breakdown is greater than synthesis, there will be a decrease in the amount of that tissue.
§ If breakdown equals synthesis, there will be no change in the amount of that tissue.
This also means that, fundamentally, we have two different ways to have an impact on the amount of a given tissue. Let’s say for example that someone wants to increase the amount of muscle that they have. They can either try to increase protein synthesis, decrease protein breakdown, or cause both to occur.
This is an important distinction because various things (such as nutrients, training, drugs) can differentially affect each process. As you’ll see in just a second, speed of digestion is one of those things and the rate of digestion of a given protein can affect protein synthesis vs. breakdown differently.
The Now Infamous Boirie Study
Back in 1997, a research group in France published the first paper on the topic of slow and fast proteins. Titled, “Slow and fast dietary proteins differently modulate postprandial protein accretion.”, this is the paper that kicked off the entire field of fast and slow proteins.
In that paper, subjects were fed either casein or whey, the two proteins found in milk (see Milk: The New Sports Drink? A Review – Research Review for more information), and blood amino acid level along with whole body protein synthesis and breakdown were measured. I’d note that both proteins were given after a morning fast with no other nutrients (carbohydrates or fat) provided. This is important because the results of this study don’t necessarily hold when other nutrients are being consumed, or someone is consuming a given protein later in the day (when other meals are still digesting).
The researchers found the following: whey spiked blood amino acid levels faster than casein, but blood amino acid levels dropped more quickly as well. Casein, in contrast, took much longer to digest, actually providing amino acids for around 8 hours to the body (you might consider that data point the next time you hear that you have to eat every three hours or your muscles will fall off, a topic I cover in Meal Frequency and Energy Balance).
I actually want to clarify that a bit since there has been a lot of confusion over what the study actually found. Both casein and whey hit the bloodstream at about the same time (about an hour in), that is, whey didn’t actually get into the system faster. However, whey spiked blood amino acid levels higher at that one hour point. Figure 1 (taken from The Protein Book) shows this.
Amino acid profile for casein vs. whey
Note that both proteins enter the bloodstream at about the same time, around the one hour mark. Whey simply spikes blood amino acids faster (before falling back to baseline levels around hour 4). Casein, in contrast raises amino acid levels to a much lower level but they are sustained for hours (in the graphic, at the 7 hour mark, blood amino acid levels were still above where they started).
So it’s not that whey gets into the system faster, it just spikes blood amino acid levels higher at the same time point (about an hour after consumption).
Now, the next bit of this study was an examination of the effects of these proteins on protein synthesis and breakdown. Basically, it was found that whey raised protein synthesis with no effect on protein breakdown while casein decreased protein breakdown without affecting protein synthesis.
Hence, whey become known as an ‘anabolic’ protein (anabolic just means making bigger things out of smaller things) and casein was an ‘anti-catabolic’ protein (catabolic means making smaller things out of bigger things, and anti-catabolic means that casein prevents that).
I’d also note that more of the whey was burned for energy (oxidized) compared to the casein.
This, of course, got taken wildly out of context to sell protein powders. However, note above I said that the research was looking at whole-body protein synthesis and breakdown. It wasn’t examining skeletal muscle per se. It’s just as logical to conclude that the whey stimulated liver protein synthesis as skeletal muscle but, of course, supplement companies don’t ever talk about that.
And with that I’m going to wrap up Part 1. In Part 2 (tune in tomorrow), I’ll talk about more recent research and some implications of speed of digestion for answering the question What are good sources of protein?
What Are Good Sources of Protein? – Speed of Digestion Part 2
Yesterday, in What are Good Sources of Protein? – Digestion Speed Part 1, I looked briefly at the issue of protein turnover and synthesis and then looked even more briefly at the now infamous Boirie study that kicked off the interest in fast and slow proteins.
Summing up, that study found that whey and casein (the two proteins found in milk) digested at different speeds, with whey being a ‘fast’ protein that spiked amino acid levels before dropping (after 3-4 hours), and casein being a ‘slow’ protein that raised amino acid levels more gradually but remaining stable for an extended period (7-8 hours).
Of more relevance, the researchers also found that the whey protein stimulated whole-body protein synthesis without much effect on protein breakdown while casein decreased protein breakdown with little effect on protein synthesis; I’d note that there was also an increase in the oxidation (burning for energy) of the whey protein. Thus whey became known as an ‘anabolic’ protein and casein an ‘anti-catabolic’ protein.
More Commentary About the Boirie Study
One other note that I didn’t mention yesterday. The researchers also looked at how each protein impacted on net leucine balance, that is how much leucine was actually stored in the body (this is used as an indicator of what’s going on with other amino acid levels).
Despite the fact that the whey actually stimulated more protein synthesis, the casein had the larger impact on leucine balance; at the end of the feeding period, the body had stored more leucine with the casein. Phrased a bit differently, it looked as if decreasing protein breakdown was more important than increasing protein synthesis in terms of whole body leucine (and therefore, protein balance).
This study essentially created an entirely new industry in the world of sports nutrition. Interestingly (or amusingly depending on your perspective), the study was interpreted variously depending on whether the company in question was selling whey or casein. Companies selling whey focused on the increase in protein synthesis; those selling casein either pointed to the increased oxidation of whey or the fact that casein had a greater impact on net leucine balance.
Various practical suggestion came out of this as well at least in the world of sports nutrition, whey protein was suggested for first thing in the morning to get aminos into the bloodstream as quickly as possible. I’d note again that this isn’t exactly the case and I realize that this is a little bit confusing. As shown in Figure of in What Are Good Sources of Protein? – Speed of Digestion Part 1 both casein and whey start to appear in the bloodstream at about the same time point; however, whey certainly raises blood amino acid levels more quickly at that point. In contrast, casein was suggested for bedtime to provide aminos throughout the fasting period to stave off muscle breakdown.
Almost without exception, whey was suggested as the best protein for after training to get aminos into the bloodstream more quickly. As noted yesterday, not only is this not true but there is emerging data (discussed in detail in The Protein Book) that fast proteins after training are not the optimal choice for promoting lean body mass gains, slow proteins or a combination of slow and fast proteins appear to be more effective. I’d refer readers back to my article on Milk: The New Sports Drink? A Review where milk outperformed soy (a fast protein) for promoting lean body mass gains.
Others suggested that the combination of whey and casein should be superior to either in isolation; the whey provides a quick hit of aminos to boost protein synthesis while the casein provided a longer source of aminos to blunt protein breakdown. In many ways, this third group would turn out to be closer to correct than either the whey-only or casein-only groups. But I’m getting ahead of myself.
But Wait, There’s More
As I also noted yesterday, one limitation of the study in question was that the protein was given without any other nutrients (carbs or fats) and were given to folks who had fasted overnight; of course it didn’t involve any type of training (which can change the dynamics of how protein is used in the body).
Frankly, the extrapolations being made about whey or casein from the study in question were poor for this (and other reasons).
Of course, later research ended up addressing some of these issues. A followup study titled “Influence of the protein digestion rate on protein turnover in young and elderly subjects.” looked at the impact of whey and casein when it was combined with carbohydrates and fats. And the differences between the two basically disappeared under these conditions.
While whey still got aminos into the bloodstream a touch more quickly, the casein meal still had the edge in terms of net leucine retention. It’s important to note that, in both of the original studies, the amount of protein given in the whey and casein group weren’t identical; the casein group got a bit more protein and that alone might have explained the greater gain in protein.
A third study provided identical amounts of casein and whey in mixed meals to either young or elderly folks; in that study the whey group came out a bit ahead in the young but way ahead in the older subjects (older here means 72 years old). This suggested that, even in the context of mixed meals, whey had a slight edge.
I’d note here that emerging research shows that older individuals respond very different to protein than younger; their muscles appear to become insensitive to protein to some degree and various interventions (such as protein pulse feeding or fast proteins) which spike blood amino acids appear to be vastly superior. In younger individuals, this doesn’t appear to be as significantly the case.
In any case, the data on the topic was clearly pretty mixed and, in the context of a mixed meal (which is how most people eat), while whey might have a slight edge over casein it was small at best.
I’d note that none of the above applies to nutrition around training, which is a topic that I’ll have to cover in a separate article. Training changes the dynamics of a many things including how protein is used by the body so the data discussed so far doesn’t really apply to that specific situation.
The Impact of Previous Meals on Digestion Speed
Finally, before moving on, a final topic that I’ve mentioned above, which is the fact that most of the studies done feed the protein to folks after an overnight fast. While this makes the studies less complicated, it doesn’t indicate what happens when a meal is being consumed while another is still digesting.
Unfortunately, this area is poorly studied, I’m not aware of any work that has examined if the fast/slow protein concept has any relevance to a meal being eaten later in the day. I’d only note again that whole food meals take much longer to digest than is often claimed, a moderate sized meal may still be digesting 5-6 hours later. Even if a ‘fast’ protein is consumed, there’s no guarantee it will still act ‘fast’ if there’s still food sitting in the gut. Again, I’m unaware of any research on this topic.
What About Other Proteins?
So far all I’ve focused on is whey and casein as these are the proteins that have been predominantly studied. Unfortunately, there is far less data on the speed of digestion of other proteins. Soy has been examined and appears to be a fast protein; note from my article Milk: The New Sports Drink? A Review that milk was found to be superior to soy protein for gains in lean body mass with training. This is thought to be due to the rapid digestion and amino acid profile of soy.
Beyond that not a tremendous amount of data exists. One researcher collected what is available and I’ve reproduced his data (originally printed in The Protein Book) in the table below
Raw Egg Protein *
Cooked Egg Protein *
Pea Protein
Milk Protein
Soy Protein Isolate
Casein Isolate
Whey Isolate
Tenderloin Pork Steak *
* Measurements marked with an asterisk should be considered as the roughest estimates as the studies used indirect measurements of protein digestion.
Clearly there is a large variety for protein digestion rates although, as noted, some of the above values should be taken as very rough estimates.
I’d note again that this has some implication for the idea that you must eat protein every three hours. With the exception of whey, where 40 grams of protein would take roughly 4 hours for complete absorption), all proteins listed would still be digesting for far longer than the magic 3 hour period.
Once again, this is getting a bit too long so I’m going to save the final bit of this discussion for Part 3, which I’ll post on Monday. In that article I’ll look at the current fascination in the sports nutrition industry with protein hydrolysates along with a few minor topics.
Whole Foods vs. Protein Powders
I finished up What are Good Sources of Protein? Speed of Digestion Part 2 with a short chart showing the estimated digestion speeds of various proteins, including some whole foods. As someone brought up in the comments, it’s unfortunate that there isn’t more data for whole foods because of the fact that, outside of athletes, most people are eating whole food protein sources, not protein powders, to obtain the majority of their daily protein.
And, in that chart, with the exception of an estimated value for tenderloin that seems impossibly high, most whole food proteins were on the slow end of the digestion scale. This actually makes perfect sense. Whole food proteins are generally contained within a matrix of connective tissue and such (e.g. think of the chewing that you have to put into eating meats such as beef, tuna, or chicken) and that alone will slow the process of digestion down. Basically, even without direct data, I’d expect most whole food proteins to be slowly digesting proteins.
Research using whole food meals find that amino acids are still be released into the bloodstream up to 5 hours after eating them; this certainly supports the idea that whole food proteins take a long time to digest. Other researchers have suggested that a given meal will maintain the body in an anabolic state for 5-6 hours so clearly whole food proteins aren’t digesting particularly quickly.
Basically, the majority of proteins that people who aren’t obsessed athletes will be eating are going to be slowly digesting proteins.
The primary exception that I’ve examined, of course, is whey protein which digests quickly; soy isolate is also a fast protein (another that I’ll mention briefly in a second is pea protein hydrolysate). Now, whey has some nice characteristics in terms of its amino acid profile (discussed in a later segment of this article), it may improve immune system function, and have other functional health benefits. Outside of athletes, life extension folks and the obsessed health types, I’m not sure that whey protein powder is going to make up a major source of protein for the majority of people.
However, this brings me in a very roundabout way to a related topic having to do with protein powders and the different forms that they come in.
Types of Protein Powder: Concentrates, Isolates and Hydrolysates
On this note, before moving on, I want to make a couple of quick comments about protein powders since, as usual, there is a lot of confusion, hype and outright lies being made about them. Quoting directly from The Protein Book:
Protein powders come in three primary forms which are isolates, concentrates and hydrolysates. Protein concentrates typically contain roughly 80% protein with 5-6% carbohydrate and fat while isolates may contain up to 90% protein. Hydrolysates are simply isolates or concentrates which have been pre-digested (digestion of protein is called hydrolysis) by subjecting them to specific enzymes. Practically speaking, you will typically pay the least for a protein concentrate, more for an isolate and the most for a protein hydrolysate. Because of the presence of free form amino acids in protein hydrolysates, they often have a more bitter taste than either concentrates or isolates.
In the last couple of years, there has been a real push by supplement companies for expensive (and often bitter tasting) hydrolysates based on the claim that they digest much more quickly than either isolates or concentrates and thus super-speed amino acids to just worked muscles.
Ignoring the question of whether faster is actually better (see below), there is the question of whether hydrolysates actually do digest significantly faster than protein isolates. Limited research is available and while one study showed that pea protein hydrolysate digested more quickly than other concentrates, this data can’t be applied to any protein except pea protein.
One study compared the digestion speed of whey and casein to their respective hydrolysates and the simple fact is that there was no significant difference in digestion speed. Quoting from the results:
The rate of gastric emptying for all solutions was found to fit an exponential pattern (r=0.92–1).Solutions were emptied at similar rates, with half-times of (mean ± S.E.M.) 21.4±1.3, 19.3±2.2, 18.0±2.5 and 19.4±2.8 min,for the whey hydrolysate, casein hydrolysate, casein and whey protein,respectively.
Basically, there was no real difference (maybe a couple of minutes faster for the hydrolysates) between whey isolate and its hydrolysate and casein and its hydrolysate.
Translation: there is no advantage to whey or casein hydrolysates in terms of digestion speed. None. Well, unless you think paying three times the price and accepting an often bitter taste is an advantage.
Which brings me in a roundabout way to the final topic of this series within the series:
Is Faster Digestion Better?
Although this question would pretty much never come up with regards to general health and nutrition, it is one that is relevant to sports nutrition (and as noted in part 2, older individuals may obtain beneifts from fast proteins). Is it better for protein to be quickly digesting or slowly digesting?
Of course the answer is context dependent and depends on what the goal is. For the majority of applications, I hope that readers get the basic idea that I think slower digesting proteins, or a mix of slow and fast are generally superior to fast proteins by themselves.
This is especially true for non-athletic applications where I think most should simply stick with whole food proteins most of the time anyhow; in the context of a mixed meal, that will mean that the proteins being consumed will digest slowly.
As I noted in What are Good Sources of Protein? Speed of Digestion Part 2, there is emerging data that older folks may benefit from spikes of amino acids in terms of offsetting age-related muscle protein breakdown. So that is clearly one exception to my general belief that slower or slow/fast mixes are best under most conditions.
Now, a recent trend in sports nutrition is to consume nutrients (carbs, protein) around the entire training bout, this often means before, during and after training. I actually spend about 35 pages discussing this issue in The Protein Book examining the most recent research and giving specific recommendations for both strength/power and endurance athletes in terms of when, how much and what to consume around training for different types of workouts. I’m not going to repeat that here, clearly.
As discussed thoroughly in that chapter, there is emerging data that a slow or mixed fast/slow protein following training is superior to a fast protein alone (I realize that this goes against what all of the supplement companies are saying but, as usual, they are taking research out of context to sell product).
Readers might simply refer back to my article on Milk: The New Sports Drink – A Review as research clearly showed that milk (a combination of whey and casein, that is a combined slow/fast protein) was superior to soy protein (a fast protein) for supporting muscle mass gains. Post-training, slow or a combination of fast and slow is simply superior to a rapidly digesting protein.
But that’s after training and folks are currently consuming protein before and during workouts these days. Under those situations, clearly a slowly digesting protein is inappropriate if for no other reason than having protein sitting in the gut digesting while you try to train is a good way to throw up.
For pre- and during-workout protein intake, I recommend a quickly digesting protein such as whey or a good soy isolate. That is one condition where, clearly, a fast digestion speed will be superior. Under most others, I feel that a slower digesting protein or at the very least a combination of slow and fast will give the best results.
There are other possible exceptions to the above, which I’ll come back to later in this series. As a primary example, some research suggests that hormonal impact of a fast protien like whey may blunt hunger better than slowly digesting proteins such as casein.
However, empirically, I can’t say I’ve seen this be the case: most report that casein (a slow protein) or milk protein isolate (a protein powder containing both whey and casein) keeps people fuller on a diet by sitting in the stomach longer. Again, I’ll come back to this in more detail when I talk about dieting.
And that’s that for speed of digestion. On Wednesday, I’ll look quickly at protein quality before moving onto the rest of the topics I outlined in the Introduction to this series.
In the last article, What are good sources of protein – Digestibility, I examined some basics of protein digestibility and presented data on the gross digestibility of varying proteins. Summing up, animal source proteins such as meat, milk and eggs show extremely high (90%+) digestion while vegetable source proteins show much lower values.
However, the efficiency of digestion alone is not the only factor which goes into answering the question What are good sources of protein?
Recently (and by that I mean the late 90′s or so), an interest in the speed of digestion and how that impacts on various aspects of human physiology has occurred. It’s turning out that proteins can digest at fairly different rates and this turns out to affect various physiological processes; the main two are protein synthesis and protein breakdown. As with the last article, I’m going to talk about these terms in brief before moving onto the main thrust of today’s article.
Because I have a lot of information to cover, I’m going to break the topic down into two parts. In Part 1 today, I need to cover a bit more background physiology and talk about the original study that kicked off the entire interest in speed of digestion. In Part 2 (tomorrow), I’ll finish up with some other recent developments. As always, all of this information can be found in a more detailed and expanded fashion in The Protein Book.
Protein Turnover: The Combination of Protein Synthesis and Protein Breakdown
Early ideas about the body held that the different tissues such as fat cells and skeletal muscle (see What Does Body Composition Mean for a little more detail) were essentially static and unchanging. This turns out to be incorrect. At any given time in the body, pretty much all of the cells in your body are undergoing a constant process of breakdown (where larger structures are broken down into smaller) and synthesis (where smaller structures are combined to make larger).
So as you sit here reading this, your fat cells are both breaking down and re-synthesizing the triglyceride (fat) stored in them. Bone is undergoing the same constant processes as well. Of course, the same holds for protein tissues.
Right this moment, your liver is breaking down and remaking various proteins, and your skeletal muscle is in a constant source of breakdown and re-synthesis. While this is actually energetically very costly, and seems wasteful, it turns out to give the human body an incredible adaptability and ability to deal with stress.
The combination of breakdown and re-synthesis is referred to, generally, as turnover. In the context of protein based tissues, this is referred to as protein turnover.
I should note that different tissues in the body break down at drastically different rates. So while liver proteins may break down and be completely re-synthesized in a number of hours, skeletal muscle is turning over more slowly. Tissues such as organs, tendons and ligaments turn over much more slowly. As you’ll see, this actually has some implications for what I’m going to talk about in just a moment.
What happens overall to a given tissue (e.g. whether it grows, shrinks or stays the same) depends on the relative rate of synthesis and breakdown. Simply:
§ If synthesis is greater than breakdown, there will be an increase in the amount of that tissue.
§ If breakdown is greater than synthesis, there will be a decrease in the amount of that tissue.
§ If breakdown equals synthesis, there will be no change in the amount of that tissue.
This also means that, fundamentally, we have two different ways to have an impact on the amount of a given tissue. Let’s say for example that someone wants to increase the amount of muscle that they have. They can either try to increase protein synthesis, decrease protein breakdown, or cause both to occur.
This is an important distinction because various things (such as nutrients, training, drugs) can differentially affect each process. As you’ll see in just a second, speed of digestion is one of those things and the rate of digestion of a given protein can affect protein synthesis vs. breakdown differently.
The Now Infamous Boirie Study
Back in 1997, a research group in France published the first paper on the topic of slow and fast proteins. Titled, “Slow and fast dietary proteins differently modulate postprandial protein accretion.”, this is the paper that kicked off the entire field of fast and slow proteins.
In that paper, subjects were fed either casein or whey, the two proteins found in milk (see Milk: The New Sports Drink? A Review – Research Review for more information), and blood amino acid level along with whole body protein synthesis and breakdown were measured. I’d note that both proteins were given after a morning fast with no other nutrients (carbohydrates or fat) provided. This is important because the results of this study don’t necessarily hold when other nutrients are being consumed, or someone is consuming a given protein later in the day (when other meals are still digesting).
The researchers found the following: whey spiked blood amino acid levels faster than casein, but blood amino acid levels dropped more quickly as well. Casein, in contrast, took much longer to digest, actually providing amino acids for around 8 hours to the body (you might consider that data point the next time you hear that you have to eat every three hours or your muscles will fall off, a topic I cover in Meal Frequency and Energy Balance).
I actually want to clarify that a bit since there has been a lot of confusion over what the study actually found. Both casein and whey hit the bloodstream at about the same time (about an hour in), that is, whey didn’t actually get into the system faster. However, whey spiked blood amino acid levels higher at that one hour point. Figure 1 (taken from The Protein Book) shows this.
Amino acid profile for casein vs. whey
Note that both proteins enter the bloodstream at about the same time, around the one hour mark. Whey simply spikes blood amino acids faster (before falling back to baseline levels around hour 4). Casein, in contrast raises amino acid levels to a much lower level but they are sustained for hours (in the graphic, at the 7 hour mark, blood amino acid levels were still above where they started).
So it’s not that whey gets into the system faster, it just spikes blood amino acid levels higher at the same time point (about an hour after consumption).
Now, the next bit of this study was an examination of the effects of these proteins on protein synthesis and breakdown. Basically, it was found that whey raised protein synthesis with no effect on protein breakdown while casein decreased protein breakdown without affecting protein synthesis.
Hence, whey become known as an ‘anabolic’ protein (anabolic just means making bigger things out of smaller things) and casein was an ‘anti-catabolic’ protein (catabolic means making smaller things out of bigger things, and anti-catabolic means that casein prevents that).
I’d also note that more of the whey was burned for energy (oxidized) compared to the casein.
This, of course, got taken wildly out of context to sell protein powders. However, note above I said that the research was looking at whole-body protein synthesis and breakdown. It wasn’t examining skeletal muscle per se. It’s just as logical to conclude that the whey stimulated liver protein synthesis as skeletal muscle but, of course, supplement companies don’t ever talk about that.
And with that I’m going to wrap up Part 1. In Part 2 (tune in tomorrow), I’ll talk about more recent research and some implications of speed of digestion for answering the question What are good sources of protein?
What Are Good Sources of Protein? – Speed of Digestion Part 2
Yesterday, in What are Good Sources of Protein? – Digestion Speed Part 1, I looked briefly at the issue of protein turnover and synthesis and then looked even more briefly at the now infamous Boirie study that kicked off the interest in fast and slow proteins.
Summing up, that study found that whey and casein (the two proteins found in milk) digested at different speeds, with whey being a ‘fast’ protein that spiked amino acid levels before dropping (after 3-4 hours), and casein being a ‘slow’ protein that raised amino acid levels more gradually but remaining stable for an extended period (7-8 hours).
Of more relevance, the researchers also found that the whey protein stimulated whole-body protein synthesis without much effect on protein breakdown while casein decreased protein breakdown with little effect on protein synthesis; I’d note that there was also an increase in the oxidation (burning for energy) of the whey protein. Thus whey became known as an ‘anabolic’ protein and casein an ‘anti-catabolic’ protein.
More Commentary About the Boirie Study
One other note that I didn’t mention yesterday. The researchers also looked at how each protein impacted on net leucine balance, that is how much leucine was actually stored in the body (this is used as an indicator of what’s going on with other amino acid levels).
Despite the fact that the whey actually stimulated more protein synthesis, the casein had the larger impact on leucine balance; at the end of the feeding period, the body had stored more leucine with the casein. Phrased a bit differently, it looked as if decreasing protein breakdown was more important than increasing protein synthesis in terms of whole body leucine (and therefore, protein balance).
This study essentially created an entirely new industry in the world of sports nutrition. Interestingly (or amusingly depending on your perspective), the study was interpreted variously depending on whether the company in question was selling whey or casein. Companies selling whey focused on the increase in protein synthesis; those selling casein either pointed to the increased oxidation of whey or the fact that casein had a greater impact on net leucine balance.
Various practical suggestion came out of this as well at least in the world of sports nutrition, whey protein was suggested for first thing in the morning to get aminos into the bloodstream as quickly as possible. I’d note again that this isn’t exactly the case and I realize that this is a little bit confusing. As shown in Figure of in What Are Good Sources of Protein? – Speed of Digestion Part 1 both casein and whey start to appear in the bloodstream at about the same time point; however, whey certainly raises blood amino acid levels more quickly at that point. In contrast, casein was suggested for bedtime to provide aminos throughout the fasting period to stave off muscle breakdown.
Almost without exception, whey was suggested as the best protein for after training to get aminos into the bloodstream more quickly. As noted yesterday, not only is this not true but there is emerging data (discussed in detail in The Protein Book) that fast proteins after training are not the optimal choice for promoting lean body mass gains, slow proteins or a combination of slow and fast proteins appear to be more effective. I’d refer readers back to my article on Milk: The New Sports Drink? A Review where milk outperformed soy (a fast protein) for promoting lean body mass gains.
Others suggested that the combination of whey and casein should be superior to either in isolation; the whey provides a quick hit of aminos to boost protein synthesis while the casein provided a longer source of aminos to blunt protein breakdown. In many ways, this third group would turn out to be closer to correct than either the whey-only or casein-only groups. But I’m getting ahead of myself.
But Wait, There’s More
As I also noted yesterday, one limitation of the study in question was that the protein was given without any other nutrients (carbs or fats) and were given to folks who had fasted overnight; of course it didn’t involve any type of training (which can change the dynamics of how protein is used in the body).
Frankly, the extrapolations being made about whey or casein from the study in question were poor for this (and other reasons).
Of course, later research ended up addressing some of these issues. A followup study titled “Influence of the protein digestion rate on protein turnover in young and elderly subjects.” looked at the impact of whey and casein when it was combined with carbohydrates and fats. And the differences between the two basically disappeared under these conditions.
While whey still got aminos into the bloodstream a touch more quickly, the casein meal still had the edge in terms of net leucine retention. It’s important to note that, in both of the original studies, the amount of protein given in the whey and casein group weren’t identical; the casein group got a bit more protein and that alone might have explained the greater gain in protein.
A third study provided identical amounts of casein and whey in mixed meals to either young or elderly folks; in that study the whey group came out a bit ahead in the young but way ahead in the older subjects (older here means 72 years old). This suggested that, even in the context of mixed meals, whey had a slight edge.
I’d note here that emerging research shows that older individuals respond very different to protein than younger; their muscles appear to become insensitive to protein to some degree and various interventions (such as protein pulse feeding or fast proteins) which spike blood amino acids appear to be vastly superior. In younger individuals, this doesn’t appear to be as significantly the case.
In any case, the data on the topic was clearly pretty mixed and, in the context of a mixed meal (which is how most people eat), while whey might have a slight edge over casein it was small at best.
I’d note that none of the above applies to nutrition around training, which is a topic that I’ll have to cover in a separate article. Training changes the dynamics of a many things including how protein is used by the body so the data discussed so far doesn’t really apply to that specific situation.
The Impact of Previous Meals on Digestion Speed
Finally, before moving on, a final topic that I’ve mentioned above, which is the fact that most of the studies done feed the protein to folks after an overnight fast. While this makes the studies less complicated, it doesn’t indicate what happens when a meal is being consumed while another is still digesting.
Unfortunately, this area is poorly studied, I’m not aware of any work that has examined if the fast/slow protein concept has any relevance to a meal being eaten later in the day. I’d only note again that whole food meals take much longer to digest than is often claimed, a moderate sized meal may still be digesting 5-6 hours later. Even if a ‘fast’ protein is consumed, there’s no guarantee it will still act ‘fast’ if there’s still food sitting in the gut. Again, I’m unaware of any research on this topic.
What About Other Proteins?
So far all I’ve focused on is whey and casein as these are the proteins that have been predominantly studied. Unfortunately, there is far less data on the speed of digestion of other proteins. Soy has been examined and appears to be a fast protein; note from my article Milk: The New Sports Drink? A Review that milk was found to be superior to soy protein for gains in lean body mass with training. This is thought to be due to the rapid digestion and amino acid profile of soy.
Beyond that not a tremendous amount of data exists. One researcher collected what is available and I’ve reproduced his data (originally printed in The Protein Book) in the table below
Protein
Absorption Rate (g/hour)
1.4
2.9
3.5
3.5
3.9
6.1
8-10
10.0
Clearly there is a large variety for protein digestion rates although, as noted, some of the above values should be taken as very rough estimates.
I’d note again that this has some implication for the idea that you must eat protein every three hours. With the exception of whey, where 40 grams of protein would take roughly 4 hours for complete absorption), all proteins listed would still be digesting for far longer than the magic 3 hour period.
Once again, this is getting a bit too long so I’m going to save the final bit of this discussion for Part 3, which I’ll post on Monday. In that article I’ll look at the current fascination in the sports nutrition industry with protein hydrolysates along with a few minor topics.
Whole Foods vs. Protein Powders
I finished up What are Good Sources of Protein? Speed of Digestion Part 2 with a short chart showing the estimated digestion speeds of various proteins, including some whole foods. As someone brought up in the comments, it’s unfortunate that there isn’t more data for whole foods because of the fact that, outside of athletes, most people are eating whole food protein sources, not protein powders, to obtain the majority of their daily protein.
And, in that chart, with the exception of an estimated value for tenderloin that seems impossibly high, most whole food proteins were on the slow end of the digestion scale. This actually makes perfect sense. Whole food proteins are generally contained within a matrix of connective tissue and such (e.g. think of the chewing that you have to put into eating meats such as beef, tuna, or chicken) and that alone will slow the process of digestion down. Basically, even without direct data, I’d expect most whole food proteins to be slowly digesting proteins.
Research using whole food meals find that amino acids are still be released into the bloodstream up to 5 hours after eating them; this certainly supports the idea that whole food proteins take a long time to digest. Other researchers have suggested that a given meal will maintain the body in an anabolic state for 5-6 hours so clearly whole food proteins aren’t digesting particularly quickly.
Basically, the majority of proteins that people who aren’t obsessed athletes will be eating are going to be slowly digesting proteins.
The primary exception that I’ve examined, of course, is whey protein which digests quickly; soy isolate is also a fast protein (another that I’ll mention briefly in a second is pea protein hydrolysate). Now, whey has some nice characteristics in terms of its amino acid profile (discussed in a later segment of this article), it may improve immune system function, and have other functional health benefits. Outside of athletes, life extension folks and the obsessed health types, I’m not sure that whey protein powder is going to make up a major source of protein for the majority of people.
However, this brings me in a very roundabout way to a related topic having to do with protein powders and the different forms that they come in.
Types of Protein Powder: Concentrates, Isolates and Hydrolysates
On this note, before moving on, I want to make a couple of quick comments about protein powders since, as usual, there is a lot of confusion, hype and outright lies being made about them. Quoting directly from The Protein Book:
Protein powders come in three primary forms which are isolates, concentrates and hydrolysates. Protein concentrates typically contain roughly 80% protein with 5-6% carbohydrate and fat while isolates may contain up to 90% protein. Hydrolysates are simply isolates or concentrates which have been pre-digested (digestion of protein is called hydrolysis) by subjecting them to specific enzymes. Practically speaking, you will typically pay the least for a protein concentrate, more for an isolate and the most for a protein hydrolysate. Because of the presence of free form amino acids in protein hydrolysates, they often have a more bitter taste than either concentrates or isolates.
In the last couple of years, there has been a real push by supplement companies for expensive (and often bitter tasting) hydrolysates based on the claim that they digest much more quickly than either isolates or concentrates and thus super-speed amino acids to just worked muscles.
Ignoring the question of whether faster is actually better (see below), there is the question of whether hydrolysates actually do digest significantly faster than protein isolates. Limited research is available and while one study showed that pea protein hydrolysate digested more quickly than other concentrates, this data can’t be applied to any protein except pea protein.
One study compared the digestion speed of whey and casein to their respective hydrolysates and the simple fact is that there was no significant difference in digestion speed. Quoting from the results:
The rate of gastric emptying for all solutions was found to fit an exponential pattern (r=0.92–1).Solutions were emptied at similar rates, with half-times of (mean ± S.E.M.) 21.4±1.3, 19.3±2.2, 18.0±2.5 and 19.4±2.8 min,for the whey hydrolysate, casein hydrolysate, casein and whey protein,respectively.
Basically, there was no real difference (maybe a couple of minutes faster for the hydrolysates) between whey isolate and its hydrolysate and casein and its hydrolysate.
Translation: there is no advantage to whey or casein hydrolysates in terms of digestion speed. None. Well, unless you think paying three times the price and accepting an often bitter taste is an advantage.
Which brings me in a roundabout way to the final topic of this series within the series:
Is Faster Digestion Better?
Although this question would pretty much never come up with regards to general health and nutrition, it is one that is relevant to sports nutrition (and as noted in part 2, older individuals may obtain beneifts from fast proteins). Is it better for protein to be quickly digesting or slowly digesting?
Of course the answer is context dependent and depends on what the goal is. For the majority of applications, I hope that readers get the basic idea that I think slower digesting proteins, or a mix of slow and fast are generally superior to fast proteins by themselves.
This is especially true for non-athletic applications where I think most should simply stick with whole food proteins most of the time anyhow; in the context of a mixed meal, that will mean that the proteins being consumed will digest slowly.
As I noted in What are Good Sources of Protein? Speed of Digestion Part 2, there is emerging data that older folks may benefit from spikes of amino acids in terms of offsetting age-related muscle protein breakdown. So that is clearly one exception to my general belief that slower or slow/fast mixes are best under most conditions.
Now, a recent trend in sports nutrition is to consume nutrients (carbs, protein) around the entire training bout, this often means before, during and after training. I actually spend about 35 pages discussing this issue in The Protein Book examining the most recent research and giving specific recommendations for both strength/power and endurance athletes in terms of when, how much and what to consume around training for different types of workouts. I’m not going to repeat that here, clearly.
As discussed thoroughly in that chapter, there is emerging data that a slow or mixed fast/slow protein following training is superior to a fast protein alone (I realize that this goes against what all of the supplement companies are saying but, as usual, they are taking research out of context to sell product).
Readers might simply refer back to my article on Milk: The New Sports Drink – A Review as research clearly showed that milk (a combination of whey and casein, that is a combined slow/fast protein) was superior to soy protein (a fast protein) for supporting muscle mass gains. Post-training, slow or a combination of fast and slow is simply superior to a rapidly digesting protein.
But that’s after training and folks are currently consuming protein before and during workouts these days. Under those situations, clearly a slowly digesting protein is inappropriate if for no other reason than having protein sitting in the gut digesting while you try to train is a good way to throw up.
For pre- and during-workout protein intake, I recommend a quickly digesting protein such as whey or a good soy isolate. That is one condition where, clearly, a fast digestion speed will be superior. Under most others, I feel that a slower digesting protein or at the very least a combination of slow and fast will give the best results.
There are other possible exceptions to the above, which I’ll come back to later in this series. As a primary example, some research suggests that hormonal impact of a fast protien like whey may blunt hunger better than slowly digesting proteins such as casein.
However, empirically, I can’t say I’ve seen this be the case: most report that casein (a slow protein) or milk protein isolate (a protein powder containing both whey and casein) keeps people fuller on a diet by sitting in the stomach longer. Again, I’ll come back to this in more detail when I talk about dieting.
And that’s that for speed of digestion. On Wednesday, I’ll look quickly at protein quality before moving onto the rest of the topics I outlined in the Introduction to this series.
Bron: What Are Good Sources of Protein? - Speed of Digestion Pt. 1 | BodyRecomposition - The Home of Lyle McDonald



Comment