Hier zal ik de volledige serie van: 'What are good sources of protein?' vermelden. Deze kan je ook op www.bodyrecomposition.com vinden.
Ik heb de belangrijkste zaken onderstreept en JA dit is het belangrijkste, ik had veel meer kunnen onderlijnen. Ik moet ook vermelden dat indien je echt een goede blik op het geheel wilt hebben, je het natuurlijk volledig moet lezen. Zo zal je beter in staat zijn om de onderstreepte zaken te nuanceren.
Wacht AUB met comments tot de volledige serie erop staat (laatste deel is de Wrap-up). Zo blijft alles overzichtelijk.
Ik heb de belangrijkste zaken onderstreept en JA dit is het belangrijkste, ik had veel meer kunnen onderlijnen. Ik moet ook vermelden dat indien je echt een goede blik op het geheel wilt hebben, je het natuurlijk volledig moet lezen. Zo zal je beter in staat zijn om de onderstreepte zaken te nuanceren.
Wacht AUB met comments tot de volledige serie erop staat (laatste deel is de Wrap-up). Zo blijft alles overzichtelijk.

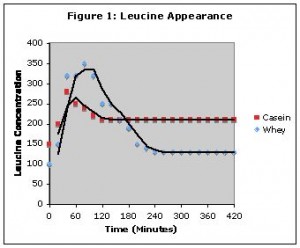
Comment